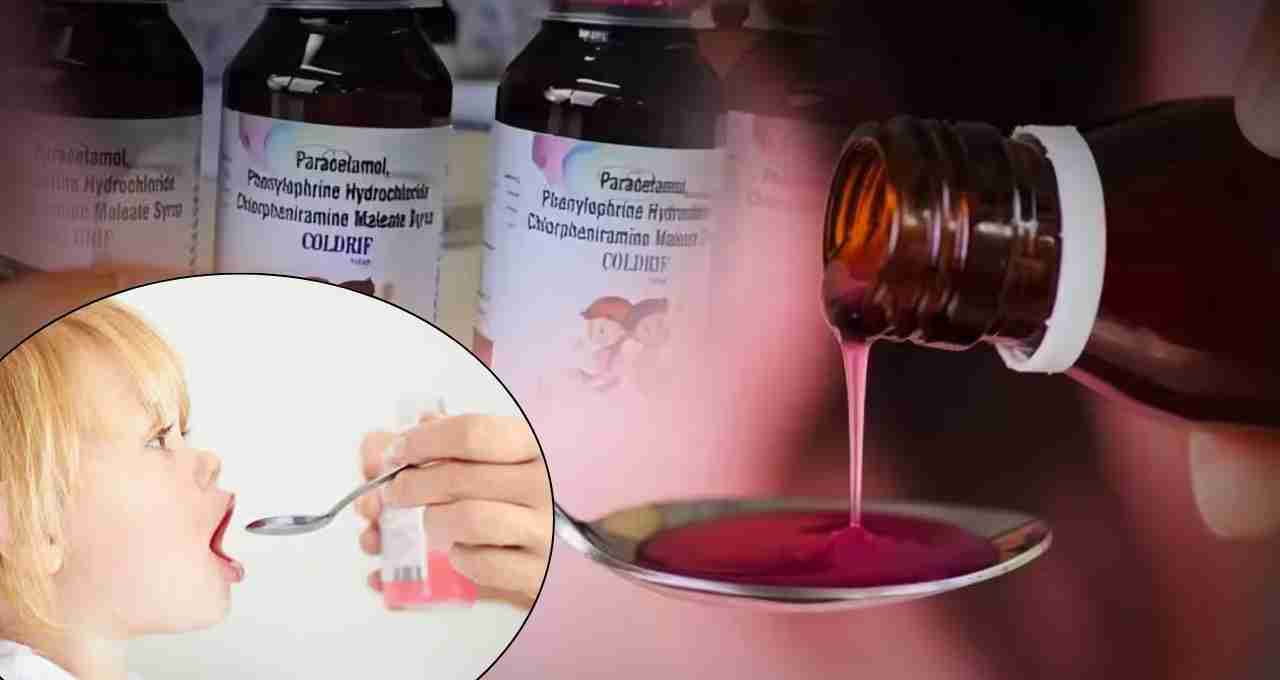
Following the deaths of children in Madhya Pradesh, the Punjab government has imposed an immediate ban on Coldrif cough syrup. Toxic diethylene glycol was found in the syrup. A complete prohibition has been enforced on its sale, distribution, and use.
Coldrif Cough Syrup Banned: Following the deaths of children in Madhya Pradesh, the Punjab government has imposed an immediate ban on Coldrif cough syrup. The Punjab Food and Drug Administration Department issued an order stating that the Madhya Pradesh government's Drug Testing Laboratory found diethylene glycol (DEG), a toxic chemical, in this syrup. The order clarified that the syrup is 'not of standard quality.' Consequently, its sale, distribution, and use are completely prohibited in Punjab.
Children's Deaths in MP
In Madhya Pradesh's Chhindwara district, several innocent children have lost their lives due to the consumption of this syrup. Investigations revealed that the syrup contained 48.6% diethylene glycol (DEG), which was causing kidney failure and severe health problems. Following this, the process of implementing safety measures has accelerated across states. Health officials and the administration have deemed it necessary to ban this syrup for the safety of children.
Public Interest Litigation in Supreme Court
Regarding the deaths of children and the sale of toxic syrup, advocate Vishal Tiwari has filed a Public Interest Litigation (PIL) in the Supreme Court. The petition demands a thorough investigation into the entire matter by the National Judicial Commission or the CBI. It also requests that the investigation be monitored by a retired Supreme Court judge to ensure fairness and transparency.
Demand for Investigation and Safety Measures
The petition specifically demands strict monitoring of the sale of toxic drugs like Diethylene Glycol (DEG) and Ethylene Glycol. Furthermore, it calls for FIRs registered in various states to be consolidated and investigated at a central level. It also demands that the licenses of companies manufacturing toxic syrups be revoked, their production immediately halted, and all products recalled from the market. Additionally, a drug recall policy should be formulated to prevent such incidents in the future.
Response from Central and State Governments
The central government has initiated risk-based inspections at 19 drug manufacturing units across six states. The National Human Rights Commission (NHRC) has issued notices to the governments of Madhya Pradesh, Rajasthan, and Uttar Pradesh, instructing immediate investigation and a halt to the sale of spurious drugs.














